Whether it’s waking up with a migraine or experiencing rapid-onset pain, patients want relief—fast.1-3

Tosymra delivers migraine pain relief in as little as 10 minutes with just one spray for some patients (13% vs. 5% for placebo)*1-3
Efficacy of Tosymra is based on relative bioavailability to subcutaneous sumatriptan at a dose of 4 mg. In a clinical study, this dose of sumatriptan resulted in 57% of patients achieving pain relief at 2 hours vs. 21% for placebo.1
*Time to onset and degree of pain relief varies by patient.
Ensure your patients are prepared
Oral therapy may not be the optimal treatment for every migraine. See why Tosymra uses the novel ingredient Intravail®.

Tosymra delivers migraine pain relief in as little as 10 minutes with just one spray for some patients (13% vs. 5% for placebo).*1-3
Efficacy of Tosymra is based on relative bioavailability to subcutaneous sumatriptan at a dose of 4 mg. In a clinical study, this dose of sumatriptan resulted in 57% of patients achieving pain relief at 2 hours vs. 21% for placebo.1
*Time to onset and degree of pain relief varies by patient.

Ensure your patients are prepared
Oral therapy may not be the optimal treatment for every migraine. See why Tosymra uses the novel ingredient Intravail®.
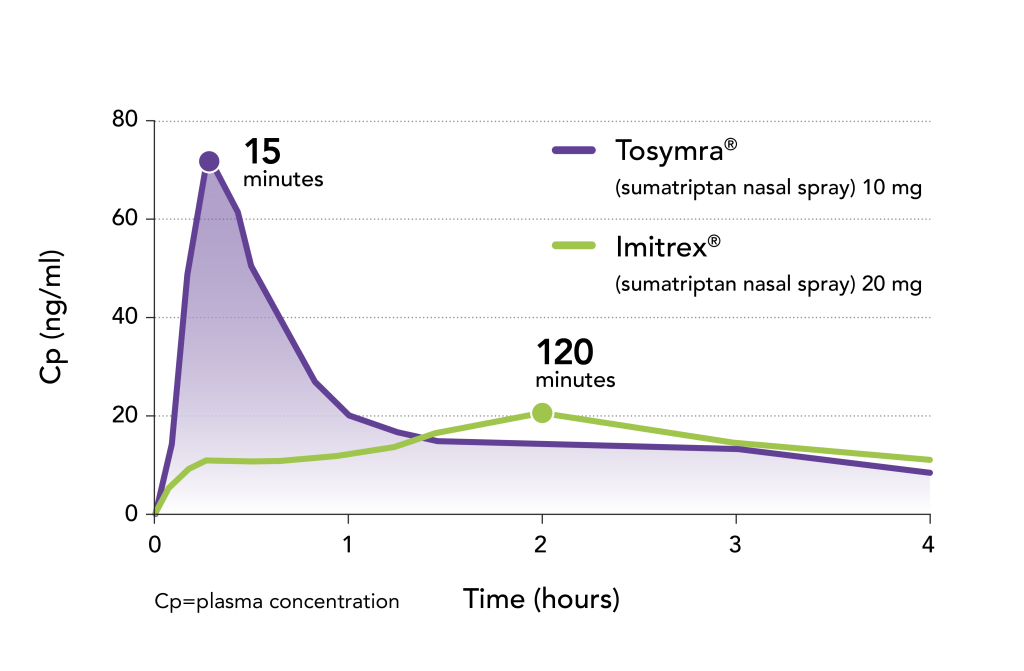
Tosymra achieved peak plasma concentration 8× faster than Imitrex® nasal spray4
Mean sumatriptan plasma concentration-time profile
- Tosymra attained 3× the peak plasma concentration (Cmax) compared with Imitrex4
- These results were obtained from a randomized, three-way crossover study comparing Tosymra with commercially available intranasal sumatriptan 20 mg (Imitrex) in 18 healthy, fasted adults
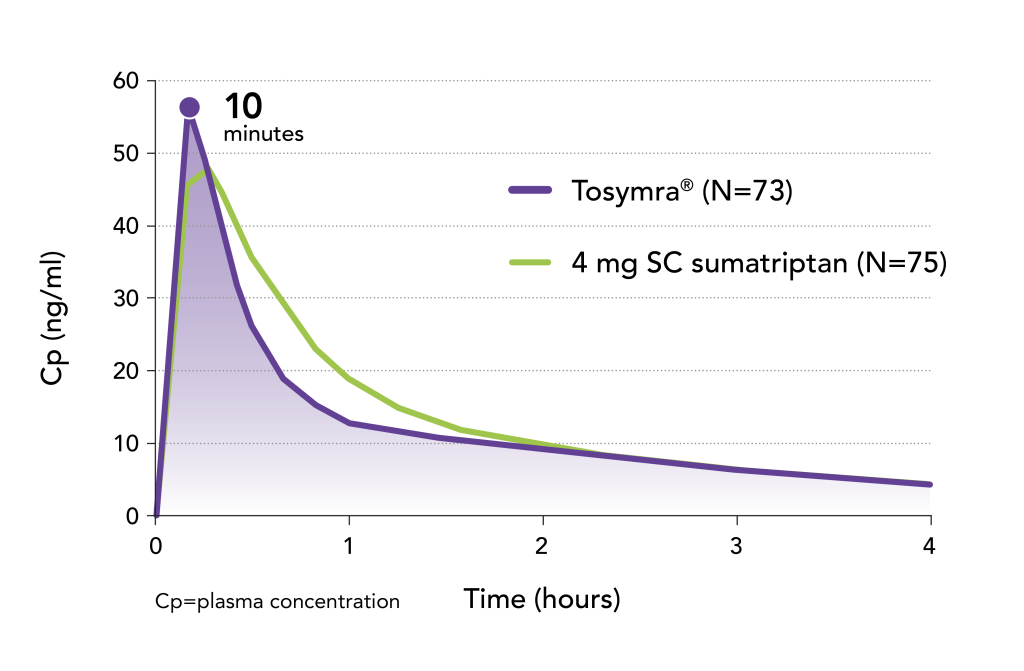
Tosymra achieved peak plasma concentration faster than 4 mg subcutaneous sumatriptan4
Mean sumatriptan plasma concentration-time profile
- Time to mean peak plasma concentration (tmax) of sumatriptan was observed 10 minutes after a dose of Tosymra, 5 minutes earlier than the subcutaneous sumatriptan dose (P <.0001)4
- These results were obtained from a open-label, randomized, single-dose, three-way crossover bioavailability study comparing Tosymra with subcutaneous sumatriptan injection in 73 or 75 patients4
Tosymra Long-Term Safety and Tolerability Study
A 6-month, open-label, repeat-dose safety study assessed the safety and tolerability of Tosymra in 167 adult patients.5 Tosymra was well-tolerated, with a low rate of treatment-emergent adverse events (TEAEs). Over the 6-month study, 3292 doses of Tosymra were used to treat 2211 migraine attacks.5
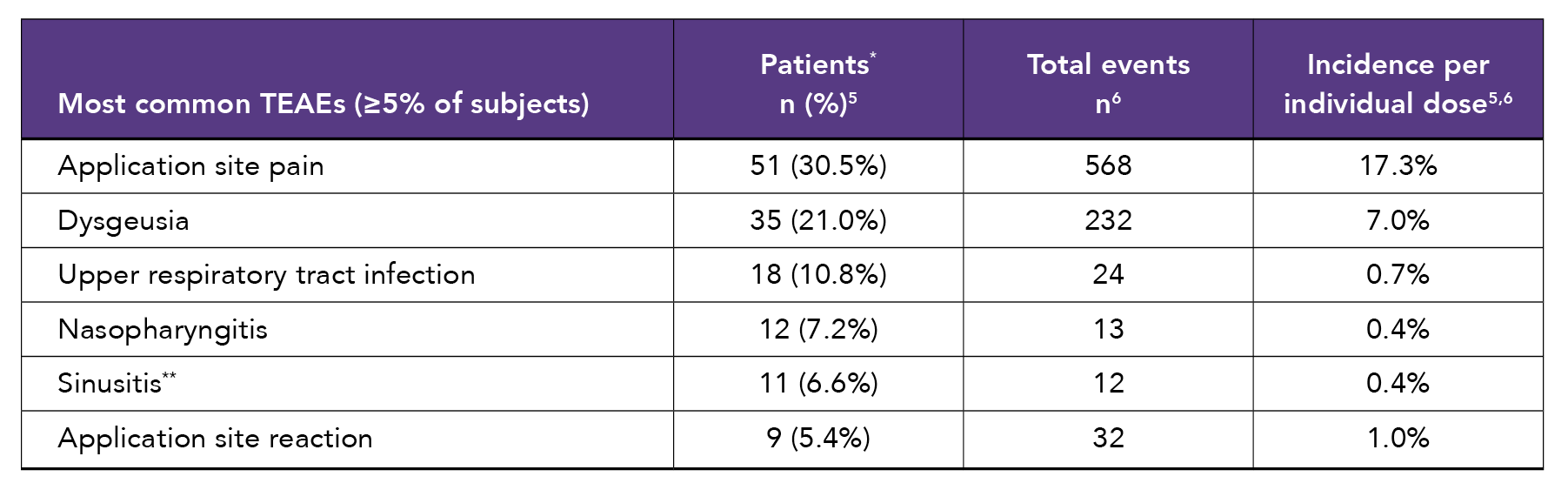
*Percent of patients experiencing the event at least once during the course of the study. †Included nasal or nostril burning or stinging. ‡Only 1 event of sinusitis was considered possibly related to Tosymra.
- Five (3%) patients discontinued due to adverse events.5
- Overall, 2.9% of doses were associated with a triptan-related TEAE.5
- Three-quarters of triptan-related adverse events were mild; none were severe.5

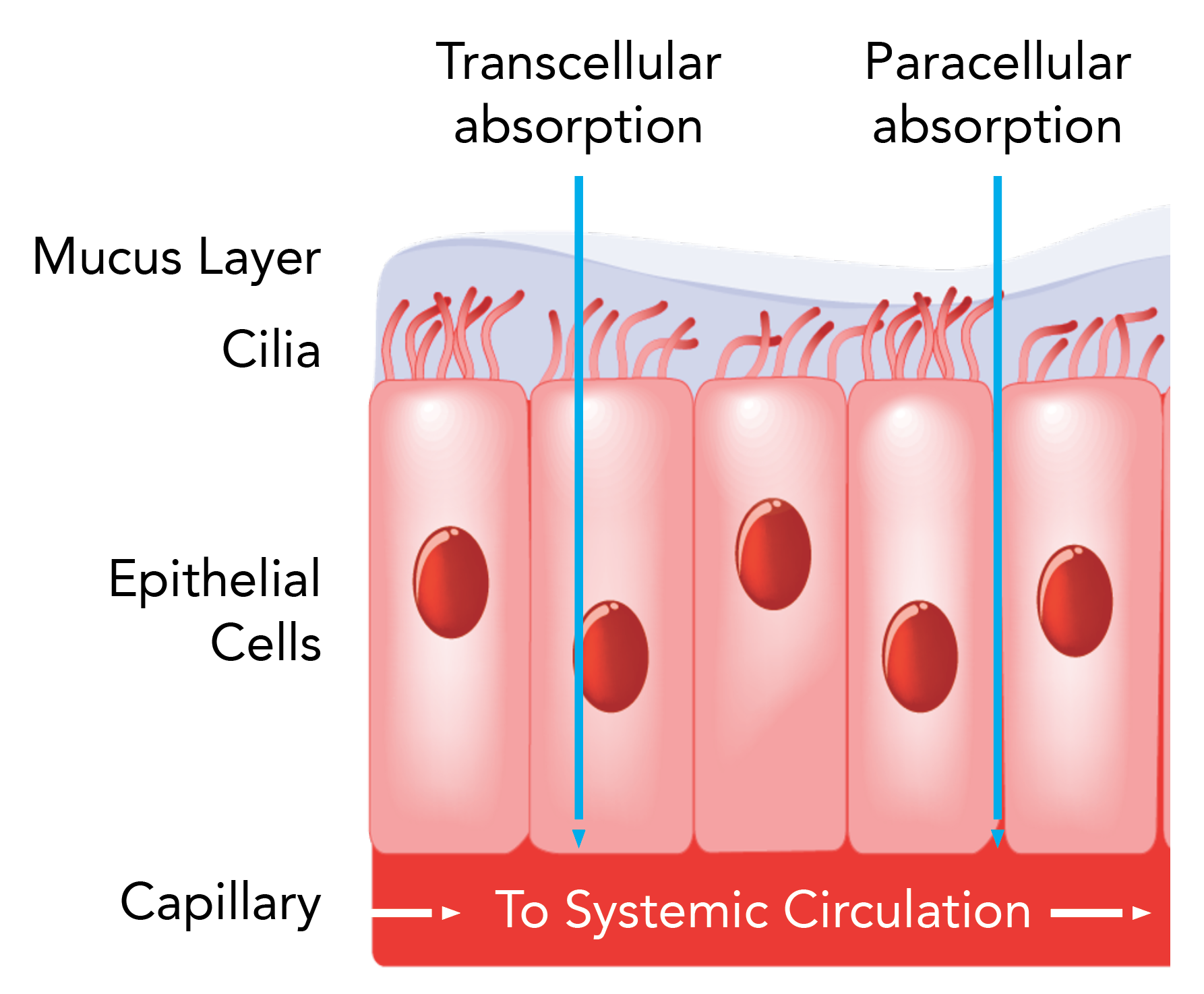
Intravail® Controlled Permeation Enhancer
Intravail (n-Dodecyl beta-D-maltoside [DDM]) is a non-toxic, odorless, and tasteless permeation enhancer that allows more medication to be absorbed through the nasal mucosa, thereby avoiding the gastrointestinal tract and first pass metabolism.7-9
Intravail®:
- facilitates paracellular absorption through the nasal mucosa by:
- transiently relaxing tight junctions between nasal mucosal cells
- facilitating drug passage into the systemic circulation of molecules up to 30 kDa, whereas the molecular weight of sumatriptan is 0.2954 kDA8
- enables Tosymra to achieve 87% of the bioavailability relative to that provided by a 4 mg subcutaneous sumatriptan injection1
- belongs to a class of compounds known as alkylsaccharides7-9
- Alkylsaccharides are used broadly as food additives and have well characterized safety and metabolic profiles8
- Alkylsaccharides metabolize quickly and cleanly to natural dietary components, namely, a sugar and a fatty acid8
- FDA designation of Generally Regarded as Safe (GRAS) for food applications8
- was non-irritating when tested at >100× the concentration used in Tosymra®7

Intravail® Controlled Permeation Enhancer
Intravail (n-Dodecyl beta-D-maltoside [DDM]) is a non-toxic, odorless, and tasteless permeation enhancer that allows the medication to bypass the GI tract, avoiding first pass metabolism8-10.
Intravail®:
- facilitates paracellular absorption through the nasal mucosa by:
- transiently relaxing tight junctions between nasal mucosal cells
- facilitating drug passage into the systemic circulation of molecules up to 30 kDa, whereas the molecular weight of sumatriptan is 0.2954 kDA8
- enables Tosymra to achieve 87% of the bioavailability relative to that provided by a 4 mg subcutaneous sumatriptan injection1
- belongs to a class of compounds known as alkylsaccharides7-9
- Alkylsaccharides are used broadly as food additives and have well characterized safety and metabolic profiles8
- Alkylsaccharides metabolize quickly and cleanly to natural dietary components, namely, a sugar and a fatty acid8
- FDA designation of Generally Regarded as Safe (GRAS) for food applications8
- was non-irritating when tested at >100× the concentration used in Tosymra®7

